Water is a unique substance in many ways, and one of its most unique properties is that it can freeze. This may seem counterintuitive at first, because most objects become denser and sink when they freeze. However, water behaves strangely. When it freezes into, it actually becomes less dense, allowing it to float. But why does this happen?
Let’s explore it.
The Structure of Water Molecules
To understand why ice density is less than water, we first need to understand the structure of the water molecule. Water (H₂O) is composed of two hydrogen atoms bonded to one oxygen atom. The atom in the molecule is about 104.5 degrees, which gives water its distinctive V-shaped structure.
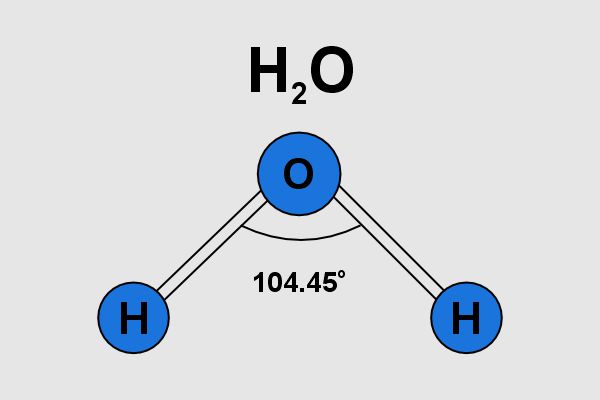
Water molecules are held together by hydrogen bonds, but still very important, hydrogen bonds. Hydrogen bonds play an important role in determining the physical properties of water, including its density.
What happens when water freezes?
When water starts to freeze, its molecules slow down and form a solid structure. In liquid water, molecules are in constant motion, sliding past each other. The hydrogen bonds are constantly being broken and re-formed, allowing the molecules to stay tightly packed but still move easily.

However, when water cools down and freezes, the molecules lose some of their energy, and the hydrogen bonds stabilize. This results in the formation of a formation of a lattice structure, where the water molecules arrange themselves in a very specific and orderly pattern. The lattice is like a 3D grid, with each water molecules positioned at a fixed distance from its neighbours.
Formation of the Ice Lattice
In the soil form of ice, the water molecules are spread out much more than in liquid water. The hydrogen bonds in the lattice force the molecules to move away from each other, creating gaps (also called “voids”) within the structure. These gaps make ice less dense than liquid water.
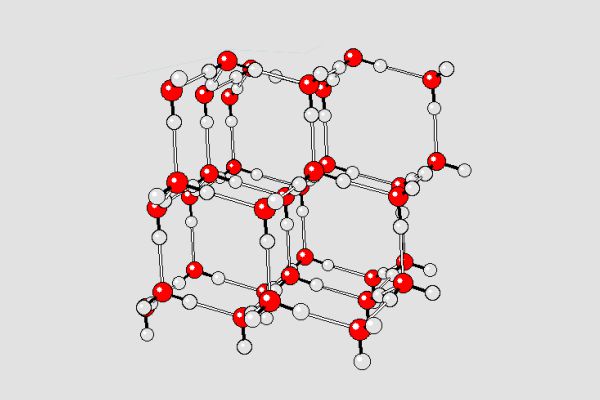
In liquid water, the molecules are close together, but because the bonds don’t break or rearrange, the molecules can pass by each other. This allows water to take up less space and therefore have a higher density.
However, in the ice lattice, the molecules are fixed in place and cannot move relative to each other. The result is a more open and spacious structure, allowing the ice to take up more space than the equivalent liquid. Since density is defined as mass per unit volume, this means that ice is less dense than liquid water.
Why Does Ice Flow on Water?
Ice floats because it is less dense than liquid water. It’s like a boat floating on water, because it is less dense than liquid, the weight of the water it displaces is greater than the weight of the boat itself. Similarly, ice displaces water i.e., heavier than it which keeps in place.

This is important property of water because it allows ice to form on the surface of bodies of water like lakes and oceans. When ice forms on a surface, it acts as an insulating layer, protecting the water below and preventing it from freezing completely. This is important for the survival of aquatic life, as the organisms in the water can continue to live beneath the ice, which remains relatively stable in temperature.
Special Property of Water
The fact that ice is less dense than liquid water is a unique property of water. Most things increase in density when they freeze, but water behaves differently. This strange behaviour has important implications for life on Earth. Without this property, lakes, rivers, and oceans would freeze from the bottom up, killing the aquatic organisms living in them.

The difference between ice and water is also why we icebergs floating. Icebergs are chunks of ice that break off from glaciers or ice sheets. Because they are less dense than liquid water, about 90% of its mass is submerged underwater and only 10% is above ground.
The Role of Hydrogen Bonds in the Behaviour of Water.
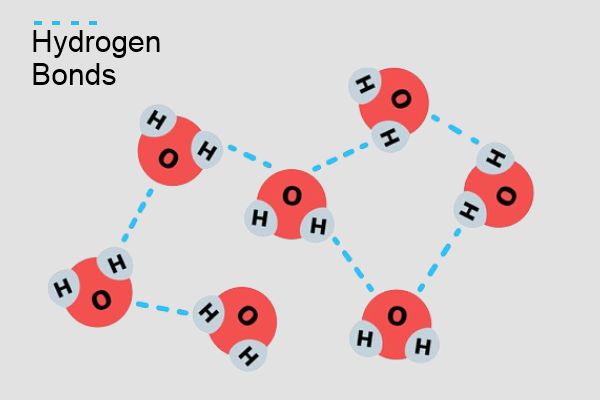
The behaviour of water and ice can be attributed to the hydrogen bonds between water molecules. These bonds are strong enough to hold the molecules together, but not strong enough to allow the molecules to move and interact with each other. When water freezes, the hydrogen bonds become more structured, forming the crystalline lattice that makes ice less dense than water.
Conclusion
In conclusion, ice is less dense than liquid water because of the structure and interaction of water molecules. When water freezes, hydrogen bonds force the molecules into an open into an open lattice structure, which creates empty spaces and increases the volume of the substance. This makes ice float on water, which is important for aquatic life and for many environmental processes.
The ability to reduce the density of water to ice is one of the fascinating aspects of water that gives it such important and unique properties. Whether it’s ice in a lake or snow on the ground, these properties have a profound effect on life on Earth.
